Journal of Animal Research: v.10 n.2, p. 181-188. April 2020
DOI: 10.30954/2277-940X.02.2020.3
Comparative Efficacy of PercollTM Discontinuous Density Gradient Centrifugation and Glass Wool Filtration Techniques for Spermatozoa Selection in Buffalo (Bubalus bubalis)
ABSTRACT
Dead and damaged spermatozoa cells present universally in the ejaculates of all eutherian mammals exert toxic effect on contemporary healthy cells mostly through generation of excessive free radicals. This is much more evident during extended period of processing, resulting in poor ejaculate quality. The solution lies in depletion of dead/damaged spermatozoa from the neat ejaculates itself. Thus the objective of the study was to evaluate the efficiency of the protocols such as discontinuous PercollTM density gradient centrifugation (PDGC) and glass wool filtration (GWF) for depletion of dead/damaged spermatozoa from fresh semen in buffalo. Random ejaculates (n=6) of Murrah buffalo bulls were divided into two aliquots after quality assessment: PDGC and GWF protocols (Group I and II, respectively). At the end of the purification protocol, efficiency of the protocols in depleting dead/damaged spermatozoa as reflected by certain quality parameters were evaluated. The mean efficiency (%) of purification protocols based on recovery of spermatozoa was 44.68 and 40.02% for PDGC and GWF, respectively. Moreover significantly (p<0.05) greater values for quality parameters was observed in the Group II (26.4+6.8 vs 68.8+4.4 for acrosome integrity (%); 12.68+6.6 vs 57.7+7.5 for functional plasma membrane integrity (%); 20.3+5.8 vs 80.75+6.7 for viability (%) in Group I and II, respectively). It was concluded that GWF is a better technique than PGDC to filter out dead/ damaged spermatozoa from fresh semen with improvement in semen quality and can be a valuable tool in assisted reproductive technology.
Keywords: Glass wool, PercollTM, Buffalo, Semen, Dead spermatozoa
Mammalian semen is a heterogeneous mixture of live as well as dead spermatozoa. It has been reported that fresh buffalo ejaculates contain 25-30% dead and damaged spermatozoa (Maurya and Tuli, 2003; Mahmoud et al., 2013; Shivahre et al., 2015). This population of dead and damaged spermatozoa in the processed semen is responsible for the production of reactive oxygen species (ROS) leading to oxidative stress responsible for poor freezability and high discard rate (Roca et al., 2013). The problem is exaggerated further in the buffalo semen due to high lipid per-oxidation rate, less activity of antioxidant system, high content of polyunsaturated fatty acids in the spermatozoa membrane and greater susceptibility to osmotic stress (Khan and Ijaz, 2008).
How to cite this article: Bisla, A., Ramamoorthy M., Rautela, R., Yadav, V., Kumar, A., Ghosh, S.K. and Srivastava, N. (2020). Comparative efficacy of percolltm discontinuous density gradient centrifugation and glass wool filtration techniques for spermatozoa selection in buffalo (Bubalus bubalis). J. Anim. Res., 10(2): 181-188.
Thus, to counter the adverse effect investigators have relied on addition of antioxidants or techniques to deplete of the dead and damaged spermatozoa from the fresh semen itself. Such spermatozoa selection techniques with varying success in buffalo include filtration techniques i.e.Sephadex filtration, glass wool filtration, gradient separation and swim up and swim down technique. The Gradient separation technique is based upon the principle of low density and inability of the dead spermatozoa to pass through the colloid suspension of varying gradient during centrifugation (Oshio, 1988). PercollTM gradient, which is a colloid solution of 15-20 nm size consisting of silica beads coated with polyvinylpyrolidone, is most commonly used in gradient separation (Pertoft et al., 1978). In glass wool filtration (GWF), the effect is provided by the changes in plasma membrane of dead and damaged spermatozoa followed by binding or agglomeration of dead and damaged spermatozoa with glass fibers (Morrell e t al., 2009). Clusterin which is negative protein, secreted from the seminal plasma and epididymal fluid, is associated with abnormal or damaged spermatozoa, and binds with glass wool to filter out dead and damaged spermatozoa (Ibrahim et al., 2001). Lee et al. (2009) observed that glass wool filtration was better technique than PercollTM discontinuous density gradient centrifugation (PDGC) for the separation of dead bovine spermatozoa. It has been observed that filtration as well as other spermatozoa selection techniques removes leucocytes as well as dead and damaged spermatozoa which are prime source of ROS in semen while selecting the morphologically normal, viable and acrosome intact spermatozoa (Anzar and Graham, 1996; Januskauskas et al., 2005). Studies regarding the comparative efficiency of GWF and PDGC of dead and damaged spermatozoa have been done in cattle and other species but the information is meager in buffalo. Therefore, the objective of the present study was to compare the efficiency of PDGC and GWF techniques as revealed by semen quality parameters in buffalo.
MATERIALS AND METHODS
Selection of the ejaculate
Six fresh semen ejaculates were collected randomly from the Murrah buffalo bulls maintained at the ICAR-Indian Veterinary Research Institute, Bareilly. The semen was collected by the artificial vagina (AV) method and after collection examined for the initial semen quality parameters. After the initial examination, the semen sample was divided into two equal parts viz., Group I (PDGC) and Group II (GWF).
Discontinuous PercollTM density gradient centrifugation (PDGC)
The PDGC was performed as per the method described by Srivastava et al. (2017) with slight modifications. Ham’s F-10 media (Table 1), BSA supplemented media (Table 2), isotonic gradient media (Table 3) and 20 and 40% (v/v) gradient media (Table 4) were prepared as fresh as per the method. Briefly, 1 mL of 40% percollTM gradient solution was layered in a 15 ml centrifuge tube with layering of 1 mL 20% percollTM gradient solution over it. 1 mL of the fresh semen ejaculate was layered over it and centrifugation was done at 400 g for 15 min. The spermatozoa pellet obtained was dissolved in 5 mL of BSA supplemented media and again centrifugation was done at 200 g for 5 min twice. The resulting spermatozoa pellet was finally dispersed in the BSA supplemented media and examined for spermatozoa concentration, livability, abnormality, acrosome intactness and functional integrity of plasma membrane.
Table 1: Composition of Ham’s F-10 medium for PDGC technique

Table 2: Composition of BSA Supplement medium for PDGC technique
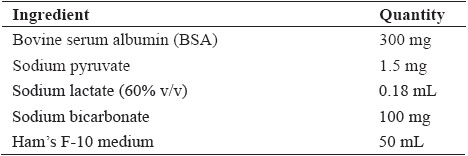
Table 3: Composition of Isotonic density gradient medium
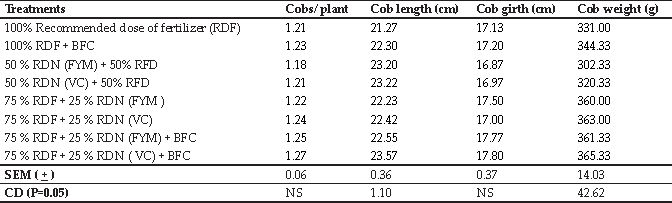
Table 4: Composition of 20% and 40% (v/v) density gradient medium
Glass wool filtration (GWF)
The GWF was performed as per the method described by Engel et al. (2001) with some modifications. Brackett and Oliphant (BO) and BO supplemented media were prepared as fresh as per the method (Table 5).
Table 5: Media composition for GWF technique
Briefly, a 5 mL capacity sterilized glass syringe was taken and filled with about 30 mg of glass wool up to 1 cm depth and multiple washing of the column were done with BO supplemented media to remove the excessive glass wool fibers coming outside the column. The assembly was maintained at 37 °C and 1 mL of 1:1 diluted fresh semen (diluted with tris buffer without egg yolk) was layered over the glass wool layer allowing filtration for 5 min. The obtained filtrate was washed with 6 mL BO supplemented media by centrifugation at 300 g for 5 min. The final washed filtrate was suspended in the BO supplemented media and examined for spermatozoa concentration, livability, abnormality, acrosome intactness and functional integrity of plasma membrane.
Semen quality parameters (SQPs)
The mass motility of the semen was assessed by putting a drop of undiluted semen on the grease free glass slide on the pre-warmed thermo-stage and was categorized from 0 to 5+ as per Salisbury et al. (1978). The spermatozoa concentration of fresh and after PDGC and GWF was determined by using Bovine photometer (Accucell, IMV technologies). The motility was recorded as percentage of progressively motile spermatozoa after the extension of small amount of fresh semen (100 μL semen with 200 μL of extender) with TYG extender and observed under high power objective (40x) of microscope fitted with thermostatically control stage after covering with a cover slip. The semen sample was extended so that approximately 15 to 20 spermatozoa were visible under the visual field of microscope (Motic, China). The individual progressive motility (IPM) was observed before and after PDGC and GWF procedure.
The spermatozoa viability and morphological abnormalities were assessed by eosin-nigrosin staining method as described by Evans and Maxwell, (1987). A drop of Eosin Nigrosin stain was taken on clean, grease free pre warmed glass slide to which one drop of semen was added, and mixed quickly but gently using a blunt fine glass rod. After 30 s to 1 min a thin smear was made on the same slide from semen and stain mixer. The smear was air dried immediately and examined under oil immersion objective of Bright field microscope (Motic, China). A total of about 200 spermatozoa were counted in each slide and per cent value calculated. The stained and partially stained spermatozoa were considered as dead/ damaged. Percentage spermatozoa morphological abnormalities were determined using same staining procedure.
Plasma membrane integrity
The spermatozoa plasma membrane integrity was determined by hypo-osmotic spermatozoa swelling test (HOST) as described by Jeyendran et al. (1984). The osmolarity of hypo-osmotic solution was kept at 150 mOsm/L. 1 mL of hypo-osmotic solution was taken in a sugar tube, to which, 0.1 mL of diluted semen was added and incubated at 37 oC in water bath. After incubation a drop of eosin-Y solution was added, small drop of the suspension from the bottom of the tube was placed on clean, grease free glass slide and covered with cover slip. A minimum of 200 spermatozoa were counted at 40X to record different types of tail swelling pattern.
Acrosome integrity
The spermatozoa acrosomal integrity was assessed by Giemsa staining of semen smears as described by Watson (1975). Briefly, a smear of diluted buffalo semen was prepared on a clean, grease free glass slide and air dried. The smear was then fixed in Hancock’s fixative for 15 min. The fixed smear was then washed in slow running water for 15 min. After drying the smear was stained in Giemsa working solution for 90-120 min. Slides containing smears were then removed from the stain solution and rinsed quickly in distilled water and air dried. The smear was then examined under oil immersion objective of the microscope to assess acrosome integrity. At least 200 spermatozoa were counted for each slide for estimation of intact acrosome percentage. The acrosome manifesting marked swelling, knobbed, ruffled, or incomplete contour and denudation were recorded as abnormal.
Efficiency of PDGC and GWF
The efficiency of PDGC and GWF techniques was calculated in comparison to the values of various semen quality parameters (SQPs) before and after the PDGC and
GWF separately as per the formula given below:
Efficiency (%) =
Statistical Analysis
The normality of data obtained from various SQPs was ascertained prior to statistical analysis. The obtained SQPs data and efficiency (%) was statistically using Graphpad Prism 8.1.2 (332) software via unpaired student’s T-test.
RESULTS AND DISCUSSION
The seminal attributes of the randomly selected samples of the Murrah buffalo bulls have been presented in Table 6.
Table 6: Seminal attributes of Murrah buffalo semen (n=6)
The mass motility of ejaculates in the present study was almost similar to Alavi-Shoushtari et al., 2009 (3.59±0.16) and Singh et al., 2013 (3.31±0.17 to 3.52±0.13) but greater than Bhakat et al., 2011 (2.88±0.02); Bhakat et al., 2015 (2.54±0.70); Shivhare et al., 2015 (2.8±0.14) and Henry et al., 2017 (2.3±1.3). The variability in the mass motility of semen could arise due to semen quality, season and microscopic examination technique of the individual observer (Bhakat et al., 2015). Mass activity of the ejaculates is also affected by spermatozoa concentration and ability of spermatozoa dislocation (Henry et al., 2017).
IPM obtained in the present study was comparable to Maurya and Tuli, 2003 (60.75±4.96); Bhakat et al., 2015 (60.64±0.02); Shivhare et al., 2015 (63.8±2.16) and Hoque et al., 2018 (64.41±14.91) but lesser than those reported by Kadirvel et al., 2009 (85.3±2.5), Lone et al., 2016 (88.25±0.36) and Lone et al., 2018 (85.27±5.25). The results of IPM are subjective and there might be difference in observation between the investigators. The procedure of semen examination, expertise of the evaluator and type of instrument used could lead to variability in findings (Bhakat et al., 2015).
Spermatozoa concentration obtained in the present study was similar to Mahmoud et al., 2013 (1079.2±21.0); Waheed et al., 2013 (1070±0.06); Bhakat et al., 2015 (1016.68±21.25) and Kumar et al., 2016 (1053.53±48.58) but lesser than the values obtained by Alavi-Shoushtari et al., 2009 (1377.14±61.22); Shivhare et al., 2015 (1749.7±122.4) and Hoque et al., 2018 (1374.31±611.29). Spermatozoa viability obtained in the present study were almost similar to Alavi-Shoushtari et al., 2009 (89.68±0.94); Kadirvel et al., 2014 (88.43±0.70) and Lone et al., 2018 (89.45±4.7) but greater than Maurya and Tuli, 2003 (70.23±2.78); Nair et al., 2006 (69.65±0.28); Bhakat et al., 2011 (67.2±0.03); Mahmoud et al., 2013 (70.9±0.7) and Shivhare et al., 2015 (77.3±2.48). The variation in the spermatozoa concentration and viability could be due to bull, age of animal, frequency of collection, false mounting before the collection, housing and feeding management as well as seasonal variation (Bhakat et al., 2015; Hoque et al., 2018).
The spermatozoa morphological abnormalities (%) obtained in the present study were lesser than Maurya and Tuli, 2003 (19.08±2.04); Alavi-Shoushtari et al., 2009 (6.53±4.07); and Shivhare et al., 2015 (6.2±0.51); Bhakat et al., 2015 (9.47±0.002) and Henry et al., 2017 (8.9±0.9). The high percentage of spermatozoa abnormalities in buffalo were found to be associated with high inbreeding of herds (Vale et al., 2008) instead of age as in other species (Saeed et al., 1990; Zorzetto et al., 2016).
The plasma membrane integrity of spermatozoa in the current study was similar to Mandal et al., 2003 (58.3±0.02) but lesser than Alavi-Shoushtari et al., 2009 (74.12); Kadirvel et al., 2014 (74.4±0.8); Shivhare et al., 2015 (75.1±1.87); Lone et al., 2016 (77.37±0.64) and Lone et al., 2018 (77.37±5.38). The acrosome integrity of spermatozoa in the current study was lesser than results of studies of Maurya and Tuli, 2003 (14.85±1.1 damaged acrosome); Alavi-Shoushtari et al., 2009 (17.35 % damaged acrosome); Kadirvel et al., 2014 (90.7±1.2); Lone et al., 2016 (86.95±0.36) and Lone et al., 2018 (88.01±3.20). The variability in plasma membrane and acrosome integrity might be due to season (Kale et al., 2000), bull, mass activity, progressive motility, spermatozoa count, total spermatozoa with intact acrosome (Prasad et al., 1999) and individual fertility level (Jeyendran et al., 1984).
The objective of the present investigation was to compare the efficiency of the PDGC and GWF protocols for removal of dead and damaged spermatozoa from buffalo semen. The comparative efficiency (%) of two protocols in terms of total and live spermatozoa recovery has been presented in Fig. 1.
Fig. 1: The efficiency (%) PDGC and GWF protocols in terms of total and live spermatozoa recovery (n=6)
The mean efficiency (%) of the two protocols (Table 7) in terms of total spermatozoa recovery vary non-significantly (p>0.05).
Table 7: The total spermatozoa concentration (million/mL) and percent recovery of spermatozoa (n=6) following PDGC and GWF techniques
Con., Concentration; Values bearing superscripts a and b differ significantly (p<0.05).
The semen quality parameters obtained after PDGC and GWF have been presented in Table 8. The obtained SQPs are similar to observations of Sherman et al. (1981) and Jeyendran et al. (1984) suggesting caution because of deleterious effects of glass wool on human spermatozoa. In contrast, Husna et al. (2016) observed that post-thaw quality of the buffalo spermatozoa improved after GWF but had no positive effect on the fertility. Panghal et al. (2002) reported effectiveness of GWF in removal of defective spermatozoa from the semen of Murrah buffalo. Filtration through glass wool has been shown to improve semen quality in cattle (Vyas et al., 1992; Anzar and Graham, 1996; Mustafa et al., 1998).
Table 8: The spermatozoa quality parameters after application of PDGC and GWF techniques (n=6)
Values bearing superscripts a and b differ significantly (p<0.05);
Values bearing superscripts x and y differ significantly (p<0.01);
Values bearing superscripts A and B differ significantly (p<0.001).
In the present investigation, spermatozoa viability (p <0.001), plasma membrane integrity ( p<0.01) and acrosome integrity ( p<0.05) obtained after GWF were significantly greater than PDGC. In agreement, Van den bergh et al. (1997) obtained better spermatozoa recovery with greater intact morphology and less contamination of selected spermatozoa with round cells and dead spermatozoa in the GWF than the PDGC.
Several authors reported selection of spermatozoa by filtration through a Sephadex column (Januskauskas et al., 2005) and separation by PDGC (Saeki et al., 1991) permitted improvements in the quality of bovine semen. However, in cases of poor semen quality (Johnson et al., 1996), high viscosity (Sakkas et al., 2003), or cryopreserved ejaculates (Coetzee et al., 1994), the technique of filtration through GWF proved to be comparatively advantageous (Engel et al., 2001). PercollTM samples have been termed as “dirty” due to presence of more debris, round cells and dead spermatozoa (Paulson and Polakoski, 1997). Vyas et al. (1992) observed that the quality of bovine semen could be improved after glass wool filtration in terms of motility, membrane integrity and a fewer abnormalities. However, free polyvinylpyrrolidone with detrimental action on the plasma membrane as well as on acrosome and mitochondrial membranes (Avery and Greve, 1995; Strehler et al., 1998) might be a major concern using polyvinylpyrrolidone based PercollTM for semen purification.
CONCLUSION
The study results show that GWF is a better technique than PGDC to filter out dead/damaged spermatozoa from fresh semen with improvement in semen quality and therefore can be preferred in techniques involving assisted reproduction in buffaloes.
ACKNOWLEDGEMENTS
The authors are thankful to Director, ICAR-Indian Veterinary Research Institute, for providing necessary facilities for conducting the research.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Alavi-Shoushtari, S.M., Rezai, S.A., Kh Ansari, M.H. and Khaki, A. 2009. Effects of the seminal plasma zinc content and catalase activity on the semen quality of water buffalo (Bubalus bubalis) bulls. Pak. J. Biol. Sci., 12(2): 134-139.
Anzar, M. and Graham, E.F. 1996.Role of sperm motility and acrosome integrity in the filtration of bovine semen. Theriogenology, 45(2): 513-520.
Avery, B. and Greve, T. 1995. Impact of PercollR on bovine spermatozoa used for in vitro insemination. Theriogenology, 44(6): 871-878.
Bhakat, M., Mohanty, T.K., Gupta, A.K., Prasad, S., Chakravarty, A.K. and Khan, H.M. 2015. Effect of season on semen quality parameters in Murrah buffalo bulls. Buff. Bull., 34(1): 100-112.
Bhakat, M., Mohanty, T.K., Raina, V.S., Gupta, A.K. and Khan, H.M. 2011. Frozen semen production performance of murrah buffalo bulls. Buffalo Bull., 30(2): 157-162.
Coetzee, K., Erasmus, E.L., Kruger, T.F., Menkveld, R. and Lombard, C.J. 1994. Glass wool filter preparation of cryopreserved spermatozoa. Andrologia, 26(1): 33-34.
Engel, S., Weber, H., Petzoldt, R., Seidl, B., Wiehe, W. and Sperl, J. 2001.An improved method of sperm selection by glass wool filtration. Andrologia, 33(4): 223-230.
Evans. G. and Maxwell, W.M.C. 1987. Salamon’s Artificial Insemination Sheep and Goats. Butterworths, Sidney.
Henry, M., Brito, M.F., Neves, B.P., Auler, P.A., Almeida, J., Andrade, G.O., Becerra, V.B. and Bergmann, L. 2017. Peculiarities of the buffalo species for andrological evaluation–results of four years of study and weekly semen collection schedule. Anim. Reprod., 14: 1225-1233.
Hoque, M.R., Rana, M.S., Nayan, S.B., Miraz, M.F.H., Deb, G.K., Nahar, T.N., Habib, R. and Siddiki, M.S.R. 2018. Influence of multiple showering on quality of buffalo semen during hot-humid season. J. Adv. Vet. Anim. Res., 5(1): 12-18.
Husna, A.U., Ejaz, R., Qadeer, S., Azam, A., Rakha, B.A., Ansari, M.S. and Akhter, S. (2016). A comparative analysis of sperm selection procedures prior to cryopreservation for Nili-Ravi buffalo bull (Bubalus bubalis) semen-: assessment of its impact on post-thaw sperm functional quality. Anim. Reprod. Sci., 174: 29-36.
Ibrahim, N.M., Foster, D.N. and Crabo, B.G. 2001.Localization of clusterin on freeze-preserved bull spermatozoa before and after glass wool sephadex filtration. J Androl., 22: 891–902.
Januskauskas, A., Lukoseviciute, K., Nagy, S., Johannisson, A. and Rodriguez-Martinez, H., 2005. Assessment of the efficacy of Sephadex G-15 filtration of bovine spermatozoa for cryopreservation. Theriogenology, 63(1): 160-178.
Jeyendran, R.S., Van der Van, H.H., Perez-Pelaez, M., Crabo, B.G. and Zaneveld, L.J. 1984. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J. Reprod. Fertil., 70: 219–228.
Johnson, D.E., Confino, E. and Jeyendran, R.S. 1996. Glass wool column filtration versus mini-Percoll gradient for processing poor quality semen samples. Fertil. Steril., 66(3): 459-462.
Kadirvel, G., Kumar, S. and Kumaresan, A. 2009. Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Anim. Reprod. Sci., 114(1-3): 125-134.
Kadirvel, G., Kumar, S., Ghosh, S. K. and Perumal, P. 2014. Activity of antioxidative enzymes in fresh and frozen thawed buffalo (Bubalus bubalis) spermatozoa in relation to lipid peroxidation and semen quality. Asia. Pac. J. Reprod., 3: 210–217.
Kale, M.M., Manik, R.S. and Tomer, O.S. 2000. Invitro assessment of crossbred buck fertility. Indian J. Anim. Sci., 70: 25-29.
Khan, M.I.R. and Ijaz, A. 2008. Effects of osmotic pressure on motility, plasma membrane integrity and viability in fresh and frozen-thawed buffalo spermatozoa. Animal, 2: 548-553.
Kumar, P., Saini, M., Kumar, D., Jan, M.H., Swami, D.S. and Sharma, R.K. 2016. Quantification of leptin in seminal plasma of buffalo bulls and its correlation with antioxidant status, conventional and computer-assisted sperm analysis (CASA) semen variables. Anim. Reprod. Sci., 166: 122-127.
Lee, H., Kim, S., Ji, D. and Kim, Y. 2009. A comparative study of Sephadex, glass wool and Percoll separation techniques on sperm quality and IVF results for cryopreserved bovine semen. J. Vet. Sci., 10: 249–255.
Lone, S.A., Prasad, J.K., Ghosh, S.K., Das, G.K., Balamurugan, B. and Verma, M.R. 2018. Study on correlation of sperm quality parameters with antioxidant and oxidant status of buffalo bull semen during various stages of cryopreservation. Andrologia., 50(4): 2970.
Lone, S.A., Prasad, J.K., Ghosh, S.K., Das, G.K., Kumar, N., Balamurugan, B., Katiyar, R. and Verma, M.R. 2016. Effect of cholesterol loaded cyclodextrin (CLC) on lipid peroxidation and reactive oxygen species levels during cryopreservation of buffalo (Bubalus bubalis) spermatozoa. Asia. Pac. J. Reprod., 5(6): 476-480.
Mahmoud, K., El-Sokary, A.A.E., Abou El-Roos, M.E.A., Abdel Ghaffar, A.D. and Nawito, M. 2013. Sperm characteristics in cryopreserved buffalo bull semen and field fertility. Iran. J. Appl. Anim. Sci., 3(4): 777-783.
Mandal, D.K., Nagpaul, P.K. and Gupta, A.K. 2003. Motion characteristics of Murrah buffalo bull spermatozoa in various seasons and its relationship with functional integrity of the plasmallema. Theriogenology, 60(2): 349-358.
Maurya, V.P. and Tuli, R.K. 2003. Post-thaw thermal resistance test on motility and acrosomal integrity of filtered and non- filtered frozen semen of Murrah buffalo bulls. AsianAust. J. Anim. Sci., 16: 1424-1428.
Morrell, J.M., Johannisson, A., Dalin, A-M. and Rodriguez- Martinez, H. 2009. Single layer centrifugation with an- drocoll-e can be scaled-up to allow large volumes of stallion ejaculate to be processed easily. Theriogenology, 72: 879- 884.
Mustafa, G., Anzar, M. and Arslan, M. 1998. Separation of motile spermatozoa from frozen thawed buffalo semen: swim-up vs filtration procedure. Theriogenology, 50: 205211.
Nair, S.J., Brar, A.S., Ahuja, C.S., Sangha, S.P.S. and Chaudhary, K.C. 2006. A comparative study on lipid peroxidation, activities of antioxidant enzymes and viability of cattle and buffalo bull spermatozoa during storage at refrigeration temperature. Anim. Reprod. Sci., 96(1-2): 21-29.
Oshio, S. 1988. Apparent densities of spermatozoa of various mammalian species. Gamete Res., 20: 159-164.
Panghal, V.S., Tuli, R.K. and Goyal, R. 2002. Influence of thawing temperature on sperm survivability on buffalo semen frozen after filtration through sephadex column. Indian J. Dairy Sci., 55: 211-213.
Paulson, J.D. and Polakoski, K.L. 1997. A glass wool column procedure for removing extraneous material from the human ejaculates. Fertil.Steril., 28: 178-81.
Pertoft, H., Laurent, T.C., Låås, T. and Kågedal, L. 1978. Density gradients prepared from colloidal silica particles coated by polyvinylpyrrolidone (Percoll). Anal. Biochem., 88: 271-282.
Prasad, J.K., Kumar, S., Mohan, G., Shanker, U. and Agarwal, S.K. 1999. Hypo-osmotic swelling test (HOST) and its response in fresh and freeze-thawed semen. Indian J. Anim. Sci., 69(10): 766-769.
Roca, J., Martinez-Alborcia, M.J., Gil, M.A., Parrilla, I. and Martinez, E.A. 2013. Dead spermatozoa in raw semen samples impair in vitro fertilization outcomes of frozen thawed spermatozoa. Fertil. Steril., 100: 875-881.
Saeed, A., Chaudhry, R.A., Khan, I.H. and Khan, N.U. 1990. Morphology of semen of buffalo bulls of different age groups. Physiol. Reprod., 3: 17-19.
Saeki, K., Hoshi, M., Leibfried-Rutledge, M.L. and First, N.L. 1991. In vitro fertilization and development of bovine oocytes matured in serum-free medium. Biol. Reprod., 44(2): 256-260.
Sakkas, D., Seli, E., Bizzaro, D., Tarozzi, N. and Manicardi, G.C. 2003. Abnormal spermatozoa in the ejaculate: abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online., 7: 428-432.
Salisbury, G.W., Van Demark, N.L. and Lodge, J.R. 1978. Physiology of Reproduction and Artificial Insemination of Cattle. San Francisco: WH Freeman & Co. pp. 428441.
Sherman, J., Paulson, D. and Liu, K. 1981. Effect of glass wool filtration on ultrastructure of human spermatozoa. Fertil. Steril., 36: 643–647.
Shivahre, P.R., Gupta, A.K., Panmei, A., Yadav, B.R., Bhakat, M., Mohanty, T.K., Kumaresan, A., Kumar, V., Dash, S.K. and Singh, S. 2015. Relationship of conventional and fluorescent microscopic technique to assess in vitro semen quality status of Murrah buffalo males. Iran. J. Vet. Res., 16: 363.
Singh, M., Ghosh, S.K., Prasad, J.K., Kumar, A., Ramteke, S.S. and Bhure, S.K. 2013. Heparin binding proteins of buffalo bulls seminal plasma and their relationship with semen freezability. Indian J. Anim. Sci., 83: 700-704.
Srivastava, N., Megha, P. and Omerdin. 2017. Semen selection protocol (In: Protocols in semen biology, comparing assays, eds. N Srivastava and Megha Pande). Springers Nature Publishing Pvt Ltd, Singapore, pp. 7-19.
Strehler, E., Baccetti, B., Sterzik, K., Capitani, S., Collodel, G., De Santo, M., Gambera, L. and Piomboni, P. 1998. Detrimental effects of polyvinylpyrrolidone on the ultrastructure of spermatozoa (Notulaeseminologicae 13). Human Reprod., 13(1): 120-123.
Vale, W.G., Ribeiro, H.F.L., Sousa, J.S., Silva, A.O.A., Barbosa, E.M. and Rolim Filho, S. 2008. Seleção e avaliaçãoandrológica do reprodutorbubalino. Rev. Bras. Reprod. Anim., 32(2): 141- 155.
Van den Bergh, M., Revelard, P., Bertrand, E., Biramane, J., Vanin, A.S. and Englert, Y. 1997. Glass wool column filtration, an advantageous way of preparing semen samples for intracytoplasmic sperm injection: an auto-controlled randomized study. Human Reprod., 12(3): 509-513.
Vyas, S., Mohan, G., Dhami, A. J. and Sahni, K. L. 1992. Comparative evaluation of the different filtration techniques for improving the semen quality of cross bred bulls. Indian J. Dairy Sci., 45: 237-240.
Waheed, M.M., Gouda, E.M. and Khalifa, T.A.A. 2013. Impact of seminal plasma superoxide dismutase and glutathione peroxidase on cryopreserved buffalo spermatozoa. Anim. Reprod. Sci., 142(3-4): 126-130.
Watson, P.F. 1975. The interaction of egg yolk and ram spermatozoa studied with a fluorescent probe. J. Reprod. Fertil., 42: 105-111.
Zorzetto, M.F., da Silva, Y.F.R.S., Zocca, S., Monteiro, G.A., Vexenat, S.C., Martin, I. and Oba, E. 2016. Physical and morphological aspects of the semen of Murrah buffaloes in sexual maturity. Vet. Zootec., 23(4): 647-655.